Executive Summary
ALCOHOLIC HEPATITIS NETWORK INTERVENTIONAL STUDY
Title of Trial
A multicenter, randomized, double blinded, placebo-controlled clinical trial of Anakinra (plus zinc) or prednisone in participants with severe alcoholic hepatitis by the AlcHepNet Consortium.
Name of Active Ingredient(s)
- Anakinra
- Prednisone
- Zinc-Sulfate
Pharmacological Class of the Drugs
- Anakinra is immunological agent, an inhibitor of the Interleukin-1 receptor (IL-1).
- Prednisone is an adrenal glucocorticoid.
- Zinc-sulfate is a nutritional supplement.
Indication
Severe alcoholic hepatitis.
Study Type
Phase 2B multicenter randomized, 2-arm, double blinded, placebo controlled clinical trial.
Investigational Sites
Indiana University, Mayo Clinic, Virginia Commonwealth University, Cleveland Clinic Foundation, University of Louisville, Beth Israel Deaconess Medical Center, University of Pittsburgh Medical Center, and University of Texas Southwestern Medical Center
Planned Number of Participants
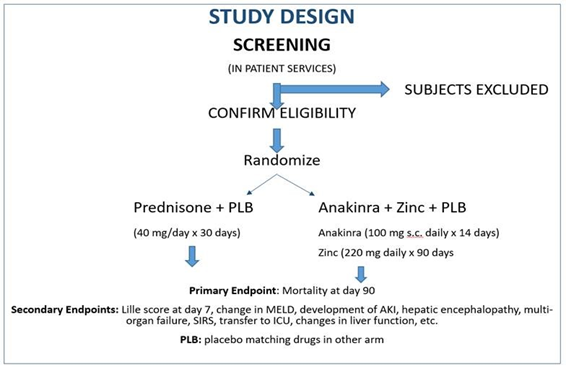
Approximately 258 participants will be randomized in a 1:1 ratio into 1 of 2 treatment arms (129 participants per arm): (1) Prednisone + Placebo for Anakinra/Zinc (2) Anakinra + Zinc + Placebo for Prednisone.
Objectives
This multicenter, randomized, double blinded, placebo-controlled clinical trial is focused on novel treatments for severe alcoholic hepatitis (AH), a life-threatening stage of alcoholic liver injury that has a short-term mortality rate much higher than that of other liver diseases.
The primary objective of the study is to determine the clinical efficacy and safety of Anakinra (plus zinc) compared to the current standard medical treatment consisting of prednisone in participants with clinically severe AH. Key secondary objectives broadly are as follows: (a) to evaluate the use of biomarkers to assess disease severity and treatment response; and (b) to develop novel endpoints to overcome the limitations of current assessment strategies for severe AH.
Overview of Study Design and Conduct
The proposed study is a Phase 2b, multicenter, prospective, randomized, 2-arm, double blinded, placebo-controlled clinical trial to assess the efficacy of Anakinra, an interleukin (IL)-1 receptor antagonist plus zinc compared to prednisone for the treatment of severe AH (Model for End Stage Liver Disease (MELD) score ≥ 20). All participants will receive standard care for treatment of severe AH as described in recent guidelines endorsed by American Association for the Study of Liver Diseases (AASLD) and American Gastroenterological Association (AGA). The study will be conducted at 8 clinical sites across the United Stated selected by the National Institute of Alcoholic Abuse and Alcoholism (NIAAA) and will be supported by a Data Coordinating Center at Indiana University (DCC-IU). The biorepository will be managed by University of Massachusetts.
The study will be conducted according to Good Clinical Practice (GCP) and in compliance with local, state and federal regulatory requirements. Adverse events during the course of the trial will be identified, recorded, assessed for causality, and reported in accordance with FDA guidance. In addition to general assessment, we will specifically focus on (1) rates and types of infection as well as their severity, (2) potential development of drug-induced liver injury, (3) injection site reactions, and (4) hematological adverse events.
This study will be approved by an appropriately convened single IRB, Western Institutional Review Board (WIRB), and will be monitored by an NIAAA appointed Data and Safety Monitoring Board (DSMB). We will conduct this study under an Investigational New Drug (IND) application from the United States Food and Drug Administration (FDA).
It is anticipated that a centrally located investigational pharmacy located at Indiana University will dispense the study medications to all participating sites under close coordination from the DCC-IU. Anakinra plus zinc, prednisone and matching placebos will be provided by the study to the participants.
Interventions to be tested
Concomitant treatments in this trial include standard care for severe AH for all participants as recommended in recent guidelines 1. The standard of care for severe AH includes attention to early diagnosis and treatment of bacterial and fungal infections, portal hypertension and its complications including acute kidney injury, gastrointestinal bleeding, fluid overload (ascites and edema) and hepatic encephalopathy.
In addition, all participants will be offered as part of the standard of care, non-pharmacologic intervention for treatment of alcohol use disorders, such as cognitive behavioral therapy and 12-step programs intervention as aligned with the best practices at the participating study site.
Participants with severe AH are very sick and often have multiple active medical problems requiring intervention. The use of specific interventions during this time has the potential to modify treatment response. Ideally, these interventions would be completely standardized. However, the standard of care varies by region, institution and the specialties involved in the care of the participant. The decision regarding treatments for these complications will be delegated to the primary physicians caring for the participants during hospitalizations and to the investigators during the ambulatory follow-up of the participants. Other than the general guidelines noted, no specific treatments for specific complications such as those noted are required. However, such treatments might include, but are not limited to the administration of IV fluids, intravenous human serum albumin, antibiotics including antifungal medications, renal replacement therapy, supplemental oxygen, assisted ventilation and intravenous pressors. Diagnostic procedures such as paracentesis, upper endoscopy, colonoscopy, endoscopic ultrasound and liver biopsy will be permitted as required to provide standard care.
During the initial six months of trial, the investigators will develop a "manual of clinical operations" that will include best practices on the use of vasopressors, albumin, ICU care, mechanical ventilation and renal replacement therapy using Surviving Sepsis Campaign, International Guidelines for Management of Severe Sepsis and Septic Shock. The investigators will also provide best practices for management of nutrition and alcohol counseling and minimization of recidivism. Although the management of these complications will not be mandated by protocol, the care provided will be recorded to develop novel designs for future trials and for practice guidance and quality improvement programs.
After informed consent has been obtained and all of the inclusion are met and none of the exclusion, participants will be randomized to receive one of the following interventions in addition to standard care.
Group 1: Standard of care plus prednisone 40 mg orally once daily on Days 1-30 and matching placebos for Anakinra (1 syringe s.c. once daily on Days 1-14), and zinc (matched pill once daily on Days 1-90).
Group 2: Standard of care plus Anakinra (100 mg s.c.) once daily on Days 1-14 zinc sulfate 220 mg once daily on Days 1-90, and placebo for prednisone (matched pill once daily on Days 1-30).
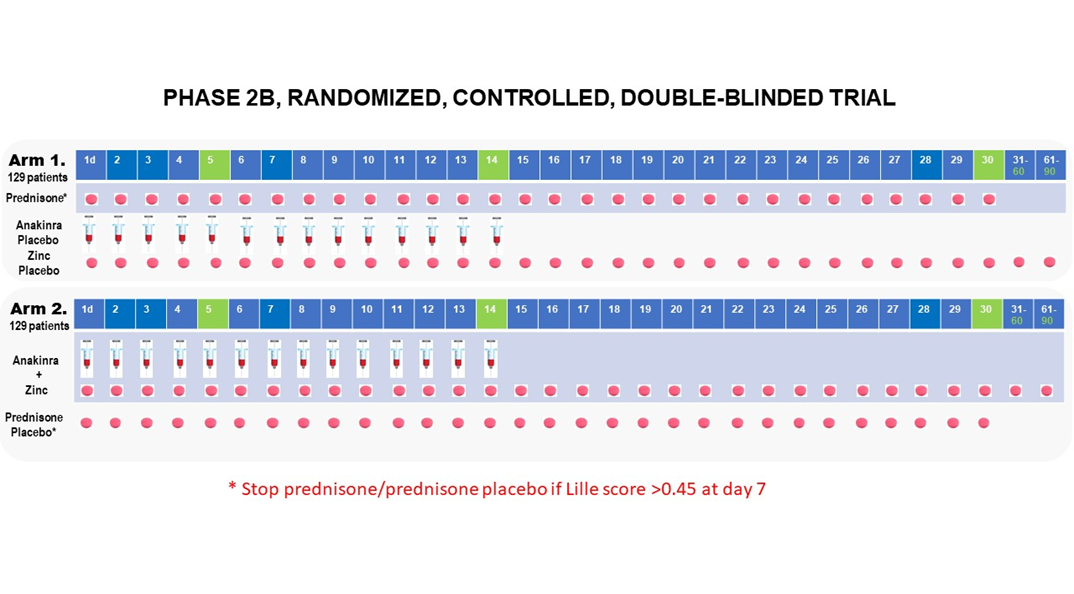
Participants or guardian will be instructed in subcutaneous injection technique to continue self-administration of these experimental treatments after discharge from the hospital. Study personnel will ensure participants and guardian are proficient in the technique before the end of study visit and an instructional document will be provided.
Primary and Important Secondary Endpoints
Primary Endpoints
Survival at 90 days
Secondary Endpoints
- Changes in score(s): Changes in Lille score, change in MELD score, development of AKI, multi-organ failure, SIRS, transfer to ICU, changes in liver function will all be evaluated at 7, 30 and 90 days.
- Organ dysfunction: Measure of changes in Sequential Organ Failure Assessment (SOFA) scores and proportions requiring hemodynamic support for MAP < 65 mm Hg and lactate > 2 mmol/l, renal replacement therapy or mechanical ventilation. The SOFA score will be modified and re-evaluated without platelet counts given that these are usually low in AH.
- Infections and Sepsis: The endpoints and case definitions related to sepsis may vary depending on what will be used (clinical care, research, surveillance or quality improvement). Given the central role of sepsis as a driver of outcomes, measuring the types of infection will occur but also identify the proportions of those with sepsis, septic shock or quick-SOFA (qSOFA) criteria based on SEPSIS-3 guidance 2, 3 . Key parameters will be captured and needed for various sepsis related endpoint construction aligned by the recent guidance from the European Drug Development Hub (http://eddh-cro.wixsite.com/fdtsfv).
- Renal dysfunction: In alignment with guidance from the acute disease quality initiative, the AKI development will be quantified and its progression through persistent AKI, acute kidney disease to chronic kidney disease.
- Need for care escalation: Proportion of participants requiring transfer to ICU for care, intubation for airway control, need for ventilator support or RRT.
- Indicators of gut permeability: (endotoxin and bacterial 18S DNA) and pro-inflammatory cytokine/chemokines (TNFa, MCP1, IL-6, IL-1ß) will be assessed in serum/plasma samples.
- Survival at 30 days and 180 days
Limitations of mortality as an endpoint in AH and plans to overcome these
All-cause mortality is most relevant when mortality is substantial and attributable to a single mechanism in a homogeneous population. Participants with severe AH are highly heterogeneous with respect to disease severity, end organ involvement and the care they receive. Moreover, as in sepsis and heart failure, death involves failure of multiple organs (liver, heart, kidney, lungs) making it difficult to ascertain which one is the primary cause of death. This has led to development and regulatory acceptance of composite endpoints including mortality and key parameters driving mortality for acute heart failure trials. These guiding principles to construct a novel and innovative key secondary composite endpoint which will include death or worsening of the modified SOFA score 4, 5 by ≥2 points and worsening of MELD score by ≥2 points. These are associated with mortality and capture the organ recruitment that marks progression of AH towards death. This endpoint will also be validated with respect to reliability, construct-, criterion- and content validity to establish this as a primary endpoint in future trials. Model changes in SOFA scores and MELD scores against mortality to further optimize this endpoint and cross-validate it in the observation cohort will occur.
Novel (experimental) endpoints to overcome limitations of conventional endpoints
Efficacy endpoints must capture "clinically meaningful benefit". While traditional endpoints such as mortality are meaningful, they also have multiple shortcomings in the context of AH as noted below. Innovation will be used to construct, measure and validate novel additional endpoints to overcome the limitations of currently used endpoints and cross-validate them in the observational cohort. The combined expertise in endpoint development and the use of harmonized measurements will make this feasible. These will provide novel assessments in AH and provide the scientific evidence-base to use these as primary endpoints in future trials.
AlcHepNet Organizations

AlcHepNet is a clinical and translational research initiative funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), a division of NIH.